
On this Page
- Summary
- What Is a CTMS?
- Why Trials Need a Clinical Trial Management System (CTMS)?
- Key Features of a Modern CTMS
- What Are the Benefits of a CTMS?
- Who Benefits from a Clinical Trial Management System (CTMS)
- How to Choose the Right CTMS?
- Getting Started with a CTMS in Clinical Trials
- Challenges in Clinical Trial Management
- The Future of CTMS
- Summary
- What Is a CTMS?
- Why Trials Need a Clinical Trial Management System (CTMS)?
- Key Features of a Modern CTMS
- What Are the Benefits of a CTMS?
- Who Benefits from a Clinical Trial Management System (CTMS)
- How to Choose the Right CTMS?
- Getting Started with a CTMS in Clinical Trials
- Challenges in Clinical Trial Management
- The Future of CTMS
Summary
A clinical trial management system (CTMS) is a software that centralizes and organizes all aspects of clinical trials. It enables sponsors, CROs, and research sites to track participants, manage sites, and monitor study progress. By integrating with other systems like EDC or RTSM, a CTMS ensures accurate and up-to-date data.
Managing a clinical trial involves coordinating multiple sites, tracking participant progress, ensuring regulatory compliance, and handling budgets and payments. Without a centralized system, these tasks can become fragmented, error-prone, and time-consuming. Trial teams often struggle to maintain real-time data visibility and seamless communication between stakeholders. That’s where a CTMS comes into play.
What Is a CTMS?
Beyond basic organization, a CTMS serves as the operational backbone of clinical research. It provides real-time dashboards for monitoring enrollment milestones, automated alerts for protocol deviations, and comprehensive audit trails for regulatory inspections. The system streamlines workflows across departments, from finance teams managing invoices to regulatory affairs tracking document submissions, ensuring everyone works from a single source of truth.
Evolution of CTMS
Early clinical trials relied on spreadsheets and homegrown solutions, which often caused redundant data entry and workflow inefficiencies. As trials grew more complex, spanning multiple countries, hundreds of sites, and thousands of participants, purpose-built CTMS platforms emerged to integrate various eClinical technologies.
Today's systems seamlessly connect with EDC, RTSM, and eTMF platforms, creating a unified ecosystem that streamlines operations and centralizes data management for sponsors, CROs, and research sites.
Why Trials Need a Clinical Trial Management System (CTMS)?
The Challenge Without CTMS
Without a dedicated management system, clinical trials face significant operational hurdles. Protocol deviations go unnoticed until monitoring visits, site performance metrics remain scattered across emails and reports, and audit preparation becomes a scramble to compile documents from multiple sources. Manual data reconciliation across disconnected systems consumes valuable time, while the lack of real-time visibility prevents proactive decision-making. These inefficiencies inflate costs and extend timelines.
The Solution a CTMS Provides
A CTMS centralizes study information and provides tools to track every step of a clinical trial by enabling:
- Unified participant enrollment and visit tracking
- Document and regulatory compliance management
- Real-time study dashboards and milestone monitoring
- Seamless integration with EDC, eTMF, and other eClinical systems
Key Features of a Modern CTMS
A modern clinical trial management system (CTMS) provides an integrated platform to support all stages of a clinical trial. While features vary across systems, the following are standard in advanced CTMS:
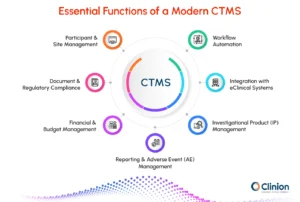
Participant & Site Management
- Track enrollment, visits, and retention
- Monitor site performance and activity
- Centralize participant records
Document & Regulatory Compliance
- Central document storage and version control
- Alerts for protocol deviations
- Audit trails and e-signatures
Financial & Budget Management
- Track budgets and payments across sites
- Automate invoices and payment schedules
- Forecast trial costs in real time
Reporting & Adverse Event (AE) Management
- Real-time dashboards and reports
- AE tracking with notifications
Investigational Product (IP) Management
- Track IP inventory and shipments
- Monitor storage conditions and expiration dates
- Ensure regulatory compliance
Integration with eClinical Systems
- Sync with EDC, RTSM, and eTMF
- Maintain consistent data across platforms
Workflow Automation
- Automate routine tasks
- Assign and track team responsibilities
What Are the Benefits of a CTMS?
Accelerated Trial Timelines
Automated workflows and real-time visibility into enrollment, site activation, and milestones help teams identify bottlenecks early and take immediate corrective action. By streamlining processes from study startup through closeout, organizations can complete trials weeks or months faster, accelerating time-to-market for critical therapies.
Reduced Operational Cost
A CTMS eliminates redundant data entry, automates invoice processing, and optimizes resource allocation across sites and vendors. Organizations report operational cost reductions of 20-30% by consolidating disparate tools into a single platform while minimizing expenses related to protocol deviations and compliance issues.
Enhanced Data Quality and Compliance
Complete audit trails automatically document every action throughout the trial lifecycle, with electronic signatures and version-controlled documents ensuring inspection readiness. This comprehensive documentation reduces audit preparation time from weeks to days while significantly lowering the risk of regulatory findings or study delays.
Improved Site Engagement and Performance
Clear communication channels and easy access to study documents, timelines, and performance metrics reduce site frustration and administrative burden. This improved experience leads to better site retention across studies, faster patient enrollment, higher protocol adherence, and more reliable trial data.
Proactive Risk Management
Automated alerts and real-time dashboards surface enrollment challenges, budget overruns, protocol deviations, and site performance issues before they become critical. This forward-looking approach enables strategic interventions such as reallocating resources, adjusting timelines, or providing site support, preventing small issues from derailing entire studies.
Scalability Across Studies
Standardized templates, reusable workflows, and centralized reporting make it efficient to launch new trials and maintain consistency across programs. Organizations can scale from managing a handful of studies to dozens or hundreds while maintaining quality and oversight across the entire portfolio.
Who Benefits from a Clinical Trial Management System (CTMS)
A CTMS delivers measurable value across every part of the clinical research ecosystem.
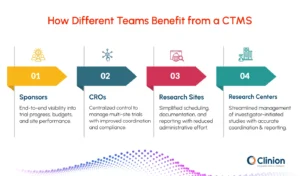
Sponsors
Gain complete visibility into trial performance, budgets, and site progress, enabling informed and timely decisions.
CROs
Coordinate multi-site studies from a unified platform, improving communication, timelines, and compliance tracking.
Research Sites
Simplify scheduling, documentation, and reporting while reducing administrative overhead.
Academic and Hospital Research Centers
Streamline investigator-initiated studies with better coordination, resource management, and reporting accuracy.
How to Choose the Right CTMS?
Selecting the right CTMS is about finding a system that fits the scale, complexity, and goals of your trials. The right platform should simplify coordination, improve visibility, and integrate seamlessly with your existing eClinical ecosystem.
Key Factors to Consider:
Feature Set
Ensure the CTMS supports core functions such as site management, study tracking, budgeting, and compliance monitoring.
Flexibility and Customization
Look for configurable workflows, reports, and dashboards that adapt to your study needs.
Ease of Use
An intuitive interface reduces training time and promotes faster adoption across teams.
Compliance and Validation
Verify that the system meets industry standards like 21 CFR Part 11 and includes built-in audit trails and e-signatures.
Integration Capabilities
The CTMS should connect smoothly with EDC, RTSM, and other eClinical systems to minimize data silos.
Support and Onboarding
Responsive support and guided implementation can significantly reduce setup time and early-stage hurdles.
Scalability and Cost
Choose a solution that grows with your portfolio, offering transparent pricing and multi-study scalability.
Getting Started with a CTMS in Clinical Trials
Implementing a CTMS successfully requires careful planning and clear steps:
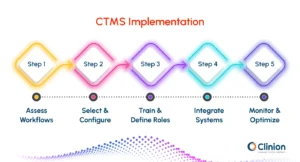
Step 1: Assess Workflows
Identify manual processes, gaps, and areas for improvement.
Step 2: Select & Configure
Choose a CTMS that aligns with trial needs and customize workflows.
Step 3: Train & Define Roles
Ensure all users understand responsibilities and system navigation.
Step 4: Integrate Systems
Connect with EDC, RTSM, eTMF, and other platforms for centralized data.
Step 5: Monitor & Optimize
Track adoption, gather feedback, and refine workflows continuously.
Challenges in Clinical Trial Management
Even with a robust CTMS in place, clinical trials face a unique set of challenges. Below are some of the key challenges that trial teams commonly encounter:
Participant Recruitment Across Sites
Coordinating enrollment and retention across multiple locations is time-consuming and resource-intensive.
Data Consistency and Quality
Ensuring accurate and standardized data across regions and teams remains a major hurdle.
Regulatory Compliance
Varying regional requirements complicate adherence and increase audit risks.
Multi-Site Coordination
Aligning communication, workflows, and timelines across multiple sites and teams can lead to delays.
System Integration
Combining multiple platforms while maintaining seamless data flow is often complex.
While these challenges persist, a well-implemented CTMS helps teams navigate them more effectively. By providing visibility, centralizing data, and supporting consistent workflows, it allows research teams to focus on maintaining study quality and meeting trial objectives across all sites.
The Future of CTMS
Clinical trials are becoming more decentralized, data-intensive, and patient-focused. To keep pace, CTMS platforms are evolving from operational tools into intelligent systems that anticipate needs, connect stakeholders, and adapt to increasingly complex study designs.
Key Developments Shaping CTMS
Predictive Analytics
Using historical and real-time data to forecast recruitment trends, site performance, and potential protocol risks.
Hybrid and Decentralized Trial Support
Integrating telehealth, remote monitoring, and wearable devices to expand reach and improve participant engagement.
Connected Ecosystem
Integration with EDC, RTSM, eTMF, and other trial systems to reduce data silos and provide a comprehensive study view.
Cloud-Based Flexibility
Secure, scalable access for global teams, enabling faster collaboration and oversight.
Participant-Centric Tools
Enhancing communication, scheduling, and adherence tracking to improve trial experiences.
CTMS platforms are moving toward greater intelligence, connectivity, and adaptability. By streamlining workflows, offering actionable insights, and supporting innovative trial designs, these systems will enable clinical teams to manage studies more effectively and maintain high standards of quality and compliance.
Clinion’s CTMS
Clinion’s CTMS centralizes trial operations, bringing together project tracking, monitoring, and financial management in a single platform. Seamlessly integrated with Clinion’s EDC and RTSM, it provides real-time visibility and operational clarity, helping CROs and sponsors maintain control and efficiency throughout the clinical trial process.

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
By providing centralized dashboards with real-time metrics, a CTMS allows project managers and sponsors to quickly identify bottlenecks, allocate resources efficiently, and adjust study plans proactively.
Yes, modern CTMS platforms support multiple therapeutic areas, offering configurable templates and workflows to meet the distinct requirements of different trial types within the same system.
Advanced CTMS platforms, including Clinion, offer native integrations with EDC and eTMF, ensuring data consistency, real-time reporting, and streamlined operational workflows across systems.
CTMS platforms store historical site data, track enrollment trends, and provide performance analytics, enabling sponsors to select the most effective sites and improve overall study outcomes.
Yes, a CTMS can track expenditures, forecast costs, automate invoice processing, and provide insights into resource allocation, helping teams manage trial finances proactively.
By centralizing patient schedules, sending automated reminders, and tracking engagement metrics, a CTMS can reduce dropouts and improve adherence across multiple sites.
CTMS platforms can flag deviations, highlight underperforming sites, and track key risk indicators, allowing teams to focus monitoring efforts where they are most needed.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


