Insights / Blog / EDC
The Ultimate Guide to Clinical Data Management for Modern Clinical Trials
- Abriti Rai
- December 18, 2025

On this Page
- Summary
- Key Objectives of Clinical Data Management (CDM)
- CDM Activities Across the Clinical Trial Lifecycle
- What is a Clinical Data Management System (CDMS)?
- Key Tools and Technologies Used in Clinical Data Management
- Roles & Responsibilities in a Clinical Data Management Team
- Essential Skills for a Career in Clinical Data Management
- Regulatory Compliance in Clinical Data Management
- The Future of Clinical Data Management: Trends & Innovations
- External Resources
- Summary
- Key Objectives of Clinical Data Management (CDM)
- CDM Activities Across the Clinical Trial Lifecycle
- What is a Clinical Data Management System (CDMS)?
- Key Tools and Technologies Used in Clinical Data Management
- Roles & Responsibilities in a Clinical Data Management Team
- Essential Skills for a Career in Clinical Data Management
- Regulatory Compliance in Clinical Data Management
- The Future of Clinical Data Management: Trends & Innovations
- External Resources
Summary
Clinical Data Management (CDM) plays a vital role in clinical trials by ensuring that trial data is reliable and meets all regulatory requirements. CDM spans the entire trial lifecycle, supporting the generation of high-quality, analysis-ready data that drives faster approvals, better decisions, and ultimately, safer therapies.
Did you know? Clinical trials generate more than 3.6 million data points per patient in Phase III studies alone, making robust clinical data management not just helpful, but essential.
Key Objectives of Clinical Data Management (CDM)

- Ensuring data accuracy and reliability: Every data point collected must reflect the true outcome of a clinical event. Inaccuracies can compromise the integrity of the entire study.
- Supporting regulatory submissions: CDM ensures that data is organized and validated according to global standards like CDISC, preparing it for successful submission to regulatory agencies such as the FDA and EMA.
- Enabling evidence-based decision-making: Reliable data allows sponsors and investigators to make confident decisions about trial progress, product safety, and efficacy.
- Ensuring patient safety: Clean, validated data support the timely identification of adverse events, enabling appropriate interventions and safeguards for participants.
CDM Activities Across the Clinical Trial Lifecycle
CDM contributes to every stage of a clinical trial, with distinct responsibilities at each phase.
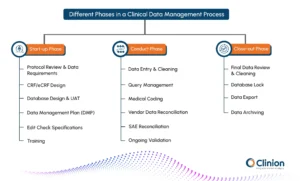
Start-up Phase
Protocol Review & Data Requirements: CDM professionals assess the protocol to identify critical variables, outcomes, and data dependencies, ensuring alignment between study design and data capture.
CRF/eCRF Design: Structured and intuitive case report forms are developed to collect protocol-specified data efficiently while minimizing ambiguity or redundancy.
Database Design & UAT: The database is configured based on CRFs and protocol needs. UAT ensures the system performs as expected and meets sponsor and regulatory requirements.
Data Management Plan (DMP): This guiding document outlines all CDM tasks, timelines, responsibilities, and risk mitigation strategies, serving as the operational blueprint for the study.
Edit Check Specifications: Logical rules are defined to detect missing, inconsistent, or illogical data at the point of entry, promoting early issue identification.
Training: Investigator sites and study teams receive training on EDC systems, query resolution procedures, and data expectations to ensure standardization across sites.
Conduct Phase
Data Entry & Cleaning: Real-time data entry occurs at the sites through the EDC system. CDM teams perform continuous data reviews to validate completeness, consistency, and accuracy.
Query Management: Queries are automatically generated or manually raised to address data issues. Efficient resolution minimizes delays and supports cleaner datasets.
Medical Coding: Terms related to adverse events, medical history, and medications are coded using standardized dictionaries like MedDRA and WHO-DD to ensure global understanding and compliance.
Vendor Data Reconciliation: Lab results, imaging, and ePRO data from external vendors are regularly matched with EDC records to prevent discrepancies.
SAE Reconciliation: Data about serious adverse events reported in safety systems is compared to clinical records to ensure accuracy and regulatory readiness.
Ongoing Validation: Interim listings, logic checks, and trend analyses are performed to proactively identify data outliers or systemic issues.
Close-out Phase
- Final Data Review & Cleaning: The CDM team conducts a rigorous final review to confirm all queries are resolved and data discrepancies addressed.
Database Lock: Once the data is deemed clean, the database is locked to prevent further changes. This is a critical milestone before final analysis.
Data Export: The locked data is exported in analysis-ready formats (e.g., SDTM/ADaM) for biostatisticians to generate trial results.
- Data Archiving: All essential records are archived in accordance with ICH-GCP and sponsor policies to support future audits or inspections.
What is a Clinical Data Management System (CDMS)?
A CDMS is a comprehensive software solution that facilitates the collection, validation, storage, and management of clinical trial data. CDMS tools provide end-to-end functionality for data entry, review, query resolution, coding, and preparation for statistical analysis and regulatory submission.
Study database creation and testing:
CDMS platforms enable the design of customized databases that reflect the unique parameters of each clinical study.
Query tracking and resolution:
In-built query modules allow data managers to track, escalate, and resolve data discrepancies across sites in a centralized environment.
Audit trail management:
Every change made in the system is tracked with time stamps and user credentials, ensuring transparency and compliance.
Integration support:
CDMS platforms support API-based integration with safety systems, labs, ePRO tools, RTSM platforms, and analytics dashboards, enabling smoother workflows.
Standardized data exports:
These systems allow seamless export of compliant datasets, reducing the time and effort needed for regulatory submission.
Key Tools and Technologies Used in Clinical Data Management
Clinical Data Management (CDM) relies on a diverse set of software tools to efficiently and accurately manage the complex data generated during clinical trials. These tools can be broadly categorized into the following key areas:

Data Collection & Capture
Electronic Data Capture (EDC) Systems:
These are the foundational tools for modern CDM. Electronic Data Capture systems replace paper-based forms with electronic ones, enabling real-time data entry, validation, and monitoring.
Key Features:
- User-friendly interfaces: Intuitive design for site staff, minimizing training requirements and reducing data entry errors.
- Robust data validation: Built-in checks for data consistency, range checks, logical checks, and other validations to minimize errors and inconsistencies.
- Workflow automation: Streamlines data flow, automates routine tasks, and ensures timely completion of data collection activities.
- Comprehensive query management: Facilitates efficient communication and resolution of data queries with investigators.
- Advanced reporting capabilities: Generate a wide range of reports for data review, monitoring, analysis, and regulatory submissions.
- Integration capabilities: Seamlessly integrate with other systems like ePRO, laboratory systems, and imaging systems.
Electronic Patient Reported Outcomes (ePRO) Systems:
Empower patients to directly input their health data, such as symptoms, quality of life assessments, and medication adherence, using electronic devices.
Key Features:
- User-friendly interfaces: Designed for easy use by patients with varying levels of technological literacy.
- Multilingual support: Ensure accessibility and inclusivity for patients across diverse populations.
- Integration with wearables: Collect objective data from wearable devices (e.g., fitness trackers, smartwatches) to provide a more comprehensive picture of patient health.
- Real-time data feedback: Provide real-time data insights to patients, investigators, and researchers, enabling timely interventions and improved patient care.
Data Management & Analysis
Clinical Data Management Systems (CDMS):
These comprehensive platforms provide a centralized repository for all clinical trial data.
Key Features:
- Data entry and management: Facilitate data entry, review, and validation.
- Data storage and retrieval: Securely store and manage all clinical trial data.
- Query management: Track and resolve data queries efficiently.
- Data reporting and analysis: Generate reports for data review, analysis, and regulatory submissions.
- Integration with other systems: Seamlessly integrate with other tools like EDC, ePRO, and laboratory systems.
Statistical Software:
Powerful tools like SAS, R, and Stata are used for statistical analysis of clinical trial data.
Key Features:
- Data manipulation and transformation.
- Statistical modeling and analysis.
- Data visualization and reporting.
Regulatory Compliance & Reporting
Safety Gateway Systems:
Facilitate the rapid reporting and monitoring of adverse events (SAEs).
Key Features:
- Automated SAE reporting: Streamline the reporting process and reduce the risk of delays.
- Signal detection and monitoring: Utilize advanced analytics to identify potential safety signals and proactively address safety concerns.
- Integration with regulatory reporting systems: Seamlessly integrate with regulatory reporting systems to streamline the submission of safety reports.
Regulatory Submission Software:
Assist in the preparation and submission of regulatory documents to agencies like the FDA and EMA.
Key Features:
- Template generation: Create and manage regulatory submission templates.
- Data extraction and formatting: Extract and format data from the CDMS for inclusion in regulatory submissions.
- Electronic submission capabilities: Facilitate the electronic submission of regulatory documents.
Trial Supply Management
Randomization and Trial Supply Management (RTSM) Systems:
Manage the randomization of patients and the distribution of investigational products to clinical sites.
Key Features:
- Complex randomization schemes: Implement intricate randomization algorithms, such as minimization and adaptive randomization.
- Supply chain management: Track drug inventory levels, manage shipments to sites, and prevent drug shortages.
- Integration with electronic prescribing: Streamline the process of prescribing and dispensing investigational products.
- Proactive risk mitigation: Identify potential supply chain disruptions and proactively address possible issues.
Data Integration & Interoperability
Data Integration Tools:
Enable seamless data exchange between different systems (e.g., CDMS, laboratory systems, EHRs).
Key Features:
- Data mapping and transformation: Translate data between different systems and formats.
- Data validation and reconciliation: Ensure data consistency and accuracy across different systems.
- Improved data flow: Streamline the flow of data between different systems, reducing manual effort and minimizing errors.
Roles & Responsibilities in a Clinical Data Management Team
Clinical Data Management is a collaborative discipline, involving multiple stakeholders with complementary skills. Each role plays a critical part in ensuring data is collected, processed, and reported in a compliant and efficient manner.
Clinical Data Manager (CDM)
- Oversees all CDM activities across the trial lifecycle, from protocol interpretation to database lock.
- Ensures data quality, regulatory compliance, and timely delivery of milestones.
- Coordinates across functional teams, including biostatistics, medical writing, and clinical operations.
Database Programmer / Clinical Database Developer
- Designs and builds study databases, including edit checks and validation rules.
- Performs unit and system testing to ensure databases are robust and error-free.
- Supports mid-study changes and data exports for analysis.
Clinical Data Coordinator / Associate
- Manages daily data cleaning activities such as query generation, tracking, and resolution.
- Performs routine data reviews to identify missing or inconsistent information.
- Acts as the first line of support for site-related data issues.
Medical Coder
- Applies standardized coding (MedDRA, WHO-DD) to medical terms and drug names.
- Ensures consistency in terminology, enabling regulatory reporting and global data interpretation.
- Maintains version control and updates for dictionary compliance.
Clinical Research Associate (CRA)
- Monitors site-level data quality and protocol adherence.
- Verifies source documents during site visits or remote monitoring.
- Collaborates with CDM on query resolution and data discrepancies.
Investigator & Clinical Research Coordinator (CRC)
- Capture and enter patient data into the EDC system at the site level.
- Ensure protocol compliance and timely responses to data queries.
- Maintain accurate and complete documentation for all study activities.
Biostatistician
- Provides input during CRF design to ensure statistical relevance of collected data.
- Analyzes cleaned data using predefined statistical models.
- Contributes to interim and final clinical study reports.
Medical Writer
- Drafts essential documents such as protocols, clinical study reports (CSRs), and investigator brochures.
- Interprets analyzed data to create accurate, compliant, and submission-ready documentation.
Insight: A successful CDM function is not just about systems; it's about collaboration. Every role contributes to generating reliable data that supports regulatory approval and patient safety.
Essential Skills for a Career in Clinical Data Management
A successful CDM professional blends domain knowledge with technical skills and collaborative capabilities. The complexity of modern trials demands more than just data entry expertise.
Technical & Domain Expertise
- Proficiency in CDMS/EDC platforms.
- Understanding of clinical trial protocols and CRF design to anticipate data capture needs.
- Knowledge of data standards, including CDISC, SDTM, ADaM, and CDASH.
Programming & Data Analytics
- Familiarity with SAS, R, Python, or SQL to manipulate, analyze, and validate large datasets.
- Ability to perform data transformations and generate data listings for review and reporting.
Regulatory & Compliance Awareness
- In-depth knowledge of ICH-GCP, 21 CFR Part 11, HIPAA, and GDPR guidelines.
- Familiarity with submission-ready formats and audit-readiness documentation practices.
Analytical & Problem-solving Abilities
- Strong critical thinking to detect inconsistencies, spot data trends, and resolve ambiguities.
- Quick decision-making during high-volume query periods or tight timelines.
Communication & Collaboration
- Ability to communicate clearly with cross-functional teams - statisticians, CRAs, medical coders, and clinical leads.
- Adept at explaining data logic or system rules to non-technical stakeholders.
Project & Time Management
- Multitasking across overlapping timelines while maintaining data quality.
- Efficient planning for interim locks, database migrations, and reconciliation deadlines.
Tip: CDM professionals who continuously upskill, especially in AI tools and data standards, will be better equipped for leadership roles in future trials.
Regulatory Compliance in Clinical Data Management
Compliance with international regulations and ethical standards is a foundational element of CDM. Every activity, from data collection to database lock, must adhere to global guidelines to ensure patient safety, data integrity, and successful regulatory submissions.
Good Clinical Practice (GCP)
- GCP outlines the ethical and scientific quality standards for designing, conducting, recording, and reporting clinical trials.
- CDM ensures that data handling procedures align with GCP to protect participants and generate credible results.
ICH Guidelines (E6 & E9)
- The International Council for Harmonisation provides critical guidance on trial conduct and statistical principles.
- CDM processes must follow ICH E6 (GCP) and ICH E9 (statistical principles) to ensure valid and reproducible outcomes.
21 CFR Part 11
- This FDA regulation governs the use of electronic records and electronic signatures.
- CDMS platforms must provide secure, audit-trailled systems with validated workflows and user access controls.
HIPAA & GDPR
- The Health Insurance Portability and Accountability Act (HIPAA) in the US and the General Data Protection Regulation (GDPR) in the EU protect patient privacy.
- CDM must implement encryption, pseudonymization, and controlled data access to safeguard sensitive patient information.
Audit Readiness & Documentation
- All CDM processes should be traceable and well-documented to support sponsor audits, regulatory inspections, and quality assurance.
- Key documents include the Data Management Plan (DMP), Edit Check Specifications, Query Logs, and Audit Trails.
Best Practice: Regular internal audits, system validations, and compliance training help maintain inspection readiness and build regulator confidence.
The Future of Clinical Data Management: Trends & Innovations
As clinical trials become more complex and data sources multiply, the CDM function is evolving rapidly. New technologies and approaches are enhancing the speed, accuracy, and scope of data management in trials.
AI & Machine Learning
- AI-driven platforms are automating repetitive tasks like query generation, SDV, and data reconciliation.
- Machine learning models are identifying data anomalies and predicting risk areas based on historical patterns.
- Natural Language Processing (NLP) is enabling automated medical coding, CSR generation, and adverse event classification.
Stat: Trials using AI-powered CDM tools have reported up to a 70% reduction in manual queries and 50% faster study closeout timelines.
Real-time Data Monitoring
- Continuous data capture and analytics dashboards allow trial teams to monitor site performance, patient safety, and data trends in real time.
- Enables faster issue identification, quicker interventions, and more agile trial management.
Integration with External Systems
- CDM systems are increasingly integrating with EHRs, lab systems, imaging platforms, and wearable devices.
- Facilitates unified datasets that support deeper insights and more comprehensive patient profiles.
Risk-Based & Remote Monitoring
- Risk-based monitoring models prioritize data review and SDV for high-risk sites and data points.
- Remote SDV tools leverage AI to reduce on-site visits by up to 50%, accelerating timelines and cutting costs.
Patient-Centric Technologies
- ePRO, eCOA, mobile apps, and wearables improve data quality while reducing site burden.
- Empower patients to contribute data more conveniently and consistently, enhancing compliance and retention.
External Resources

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
Clinical Data Management is the process of collecting, validating, and managing clinical trial data to ensure its integrity, quality, and regulatory compliance.
CDM spans three core phases: study start-up (protocol review, CRF design, database setup), conduct (data entry, query resolution, validation), and close-out (final review, database lock, data export).
A CDMS is a software platform used to build, validate, manage, and lock clinical trial databases. It includes tools for EDC, query management, coding, reporting, and audit trails.
Key skills include data handling, knowledge of regulatory standards (GCP, CDISC), software proficiency (EDC, SAS), problem-solving, communication, and attention to detail.
User Acceptance Testing (UAT) is the process of validating the clinical database and eCRFs with end users to ensure all functionalities meet study requirements before going live.
The Clinical Data Manager ensures data quality, resolves discrepancies, manages timelines, and ensures that data are clean, reliable, and ready for statistical analysis and regulatory submission.
Medical coding involves converting medical terms and adverse events into standardized codes (like MedDRA or WHO-DD) to enable uniform reporting and interpretation across regulatory regions.
AI accelerates query management, automates SDV, improves data cleaning, and enables predictive risk modeling, reducing timelines and improving data quality across trial phases.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


